-

- Research &Development
- AC-1101
AC-1101
AC-1101
AC-1101 is a proprietary topical gel formulation of the small molecule Janus kinase (JAK) inhibitor being developed for the treatment of autoimmune inflammatory skin diseases. JAKs are intracellular tyrosine kinases associated with type I and II cytokine receptors and responsible for transmitting signals in response to cytokines on the cellular membrane. Upon cytokine binding to its corresponding receptor, JAKs phosphorylate and activate the transcription factor, Signal Transducers and Activators of Transcription (STAT) to regulate a complex network of gene expression. In the last decade, JAK-STAT pathway emerged as important therapeutic target for inflammatory and autoimmune diseases due to its fundamental role in immunology and disease etiology. Currently, JAKi have been approved in the United States or worldwide to treat rheumatoid arthritis, myelofibrosis, psoriatic arthritis, ulcerative colitis, ankylosing spondylitis, vitiligo, alopecia areata, and atopic dermatitis.
AC-1101 is designed to enhance skin penetration with its proprietary formulation and to effectively modulate JAK-STAT signaling mediated by key cytokines that are involved in a variety of skin diseases including vitiligo, atopic dermatitis, granuloma annulare and rare autoimmune skin diseases. AC-1101 has the advantage in long-term use by reducing the risk of systemic side effects, commonly associated with oral JAKi.
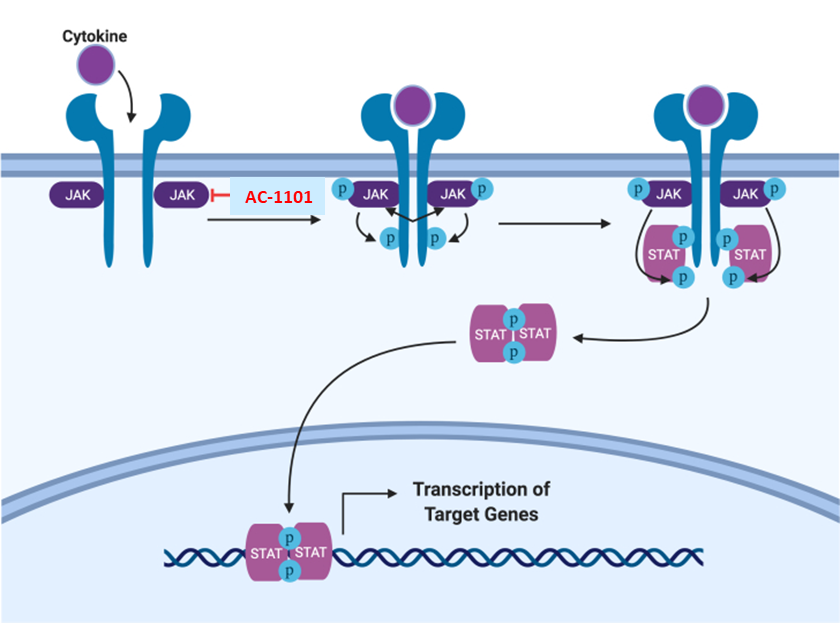
Adapted from Yale J Biol Med. 2020 Mar 27;93(1):187-195
● Drug Development
-
Apr. 2020, US FDA IND Approval for vitiligo
-
Nov. 2020, Completed Phase I PK and safety study
-
Jul. 2022, US FDA IND Approval for granuloma annulare
-
Nov. 2022, Initiated Phase Ib trial in patients with granuloma annulare
● Trial Registration
clinicaltrials.gov Identifier:
Phase I PK and safety study (AC-1101-PK-001): NCT04468425
GA phase I study (AC-1101-GA-001): NCT05580042
